Vitamin D intracrine and paracrine signaling - illustrated tutorial
Robin Whittle rw@firstpr.com.au 2022-05-15
(First established for "autocrine" signaling on 2020-11-23.)
../ To the main page of this site.
For a comprehensive overview of vitamin
D and the immune system and of the need for proper (e.g. 0.125 mg 5000
IU /day or more, for 70 kg bodyweight) vitamin D3 supplementation,
please see:
https://vitamindstopscovid.info/00-evi/ .
Introduction
Vitamin D based intracrine and
paracrine signaling are the two primary (perhaps sole) ways most immune
cells use the vitamin D compounds. These signaling systems are
crucial to the ability of each individual cell to respond to its
changing circumstances.
The immune system is second only in complexity to the nervous
system. Its operation is not coordinated by neurons. All
coordination is done by individual cells, of multiple types, sensing
their surroundings, sending chemical signals to other cells and so
changing their behaviour in various ways. Vitamin D based
intracrine and paracrine signaling is a long-evolved, flexible,
powerful (can up and down regulate the transcription of hundreds of
genes) mechanism which plays a very large role in how immune cells
change their behaviour. This only works to the extent that
sufficient 25-hydroxyvitamin D is supplied to these cells, all over the
body.
All current research indicates that 50 ng/mL 125 nmol/L
25-hydroxyvitamin D in the bloodstream provides sufficient
25-hydroxyvitamin D for immune cells. Without substantial recent
UV-B skin exposure or proper vitamin D3 supplementation (or, for
emergency repletion, calcifediol, which is 25-hydroxyvitamin D) most people have only 1/2 to 10th of this. So their immune system does not work very well.
Vitamin D based intracrine signaling is also, incorrectly, known by the
rather similar term "autocrine", but to our knowledge, there is no
vitamin D based autocrine signaling, since that would only occur if the
vitamin D receptor (VDR) molecules were located in the cell membrane
with their active site pointing outwards.
VDR is not a membrane based receptor. Intracrine signaling is
like autocrine signaling but the receptor is in the cytosol. The
previous version of this page referred to "autocrine" signaling, and I
made this new "intracrine" version on 2022-05-14.
Terminological
note 2022-05-14:
- Autocrine signaling involves some molecules (acting as an autocrine agent, not a hormone)
being made inside a cell, to signal information to another part of the
cell. The molecules leave the cell's cytoplasm and activate receptors
on the outside of the cell membrane, of the same cell.
The activated receptors cause changes inside the cell, such as changes
leading to altered transcription of genes, which leads to different
proteins being made and so to altered cellular behaviour.
This does not occur with the vitamin D compounds, in which
1,25-dihydroxyvitamin D (calcitriol) acts as a signaling molecule to
activate vitamin D receptor molecules, because these vitamin D receptor
molecules are not found on the outside of the cell's membrane.
Autocrine signaling is known to exist with other compounds and the term autocrine signaling has been used, incorrectly, to refer to what is actually intracrine signaling. This page uses "autocrine" in this way, as does Chauss et al. 2021 in their extraordinarily important work with Th1 lymphocytes from the lungs of hospitalised COVID-19 patients.
- Intracrine signaling
refers to signaling molecules being generated inside a cell where they
activate receptor molecules, also inside the same cell, with those
activated receptors altering the cell's behaviour as just
described. These molecules are acting as an intracrine agent, not a hormone.
This does occur with
1,25-dihydroxyvitamin D. As best I can tell, this signaling
system was first discovered by Martin Hewison and colleagues in the mid
to late 2000s. See below for a 1991 article entitled Intracrinology.
The exact location of the receptor molecules is important for molecular
biology research, but is a fussy detail from the point of view of most
doctors and lay people in understanding the importance of good supplies
of 25-hydroxyvitamin D to all such cells, which (in particular
circumstances) initiate their intracrine signaling system by converting
this to 1,25-hydroxyvitamin D.
#fletcher
For a recent review article on vitamin D based intracrine signaling:
My Twitter-brief appreciation of this: 1,
2,
3,
4,
5 and
6.
(I disagree with the use of "hormonal" in the abstract - this
1,25-dihydroxyvitamin D is acting as an intracrine or paracrine agent,
not as a hormone.)
- Paracrine signaling
involves a signaling molecule (in the case of vitamin D based paracrine
signaling, 1,25-dihydroxyvitamin D) being produced inside one or
typically multiple cells in a particular location in the body, due to
particular circumstances being detected by those cells, and these
molecules, acting as a paracrine agent, not a hormone,
diffuse out of the cell and find their way to nearby cells (typically
of a different type, as best I understand it) where those cells'
behaviour is changed by the presence of these diffused signaling molecules.
I guess, in humans, that "nearby" means fractions of a millimetre to a
few millimetres. I am not aware of anyone describing observed or
theoretical distances. A single cell, or multiple cells of the
same
type, may, when they detect particular circumstances, convert
intracellular 25-hydroxyvitamin D into 1,25-hydroxyvitamin D, which
acts both as an intracrine agent for the cell in which it was produced,
and as a paracrine agent when some of these molecules diffuse out of
this cell, and affect the behaviour of other nearby cells.
Vitamin D based intracrine and paracrine signaling is unrelated to the one hormonal function of vitamin D, in which a very low, but tightly regulated, concentration of circulating 1,25OHD is produced by the kidneys to control calcium-phosphate-bone metabolism.
Most people - including many doctors, immunologist and virologists
- are not familiar with intracrine or paracrine signaling. To
understand vitamin D
in general - and especially to understand why population-wide vitamin D
repletion targeting at least 50 ng/mL 25(OH)D vitamin D blood levels (125 nmol/L) is the key to improving health - we need a good understanding of vitamin D based intracrine signaling.
As far as I know, all this is correct, since it is based on some
earlier text which met with the approval of a senior vitamin D
researcher. If you spot any errors or can suggest any other
improvements, please let me know.
Contents
Sections 00 to 04 are preliminaries. To go straight to the explanation of vitamin D based intracrine signaling:
#05-intra .
#00-term
|
Notes on terminology. Read this first to reduce later confusion.
|
| #01-compounds |
D3 cholecalciferol, 25(OH)D calcifediol and 1,25(OH)2D calcitriol.
|
| #02-nothorm |
Hormonal 1,25(OH)2D for calcium-phosphate-bone metabolism is at a much lower level than the 1,25(OH)2D generated as an intracrine agent and/or paracrine agent in
immune and other cells, so the hormonal 1,25(OH)2D levels, which are quite
stable, have no significant effect on the intracrine and paracrine
signaling systems of numerous types of cell.
|
| #03-minlev |
Numerous reasons why we should aim for at least 50 ng/mL (125 nmol/L) 25(OH)D blood levels.
|
| #04-quraishi |
2014 research which indicates we should aim for at least 50 ng/mL 25(OH)D blood levels.
|
| #05-intra |
Description
of intracrine signaling with two examples from research articles, the
second of which is directly relevant to severe COVID-19.
Paracrine signaling is easily understood as an extension of this to
nearby cells, by diffusion.
|
#00-term
Confused and confusing terminology, including: "Vitamin D" "hormone" and
"autocrine" and "intracrine" signaling
Here is my best attempt at untangling some contradictory and/or divergent and at least
confusing terminological problems. This includes my own
value judgments on how particular terms should be used, and how they are sometimes misused.
The term
Vitamin D is generally and
properly used to refer
collectively to the three compounds best known in mammalian biology:
- Vitamin D3 cholecalciferol. [WP] Shorter forms: D3. This never acts as a hormone.
- 25-hydroxyvitamin D calcifediol. [WP] Shorter forms: 25(OH)D, 25OHD3, 25(OH)D3 and 25D. This never acts as a hormone. An alternative name for this is calcidiol - I suggest that this term be avoided entirely because it is redundant, confusing and looks and sounds very much calcitriol (and like calcidol which sometimes used to refer to D2 ergocalciferol, as described below).
- 1,25-dihydroxyvitamin D calcitriol. [WP] Shorter forms: 1,25(OH)2D and 1,25(OH)2D3.
This has a much greater affinity for the vitamin D receptor than D3 or
25OHD or any other vitamin D related compounds. It is sometimes
referred to as activated vitamin D but I regard this as a mistake. This has one hormonal function. All its other functions, in
probably hundreds of cell types, are not related to hormonal signaling -
it is produced and sensed by the same cell in intracrine
signaling and diffuses to nearby cells where it is sensed, in paracrine
signaling.
Sometimes "vitamin D" is used to refer just to D3, and "vitamin D
metabolites" to the other to compounds, as well as to other compounds
not mentioned here such as those which result from the breakdown of any
of the above three compounds or their hydroxylation, such as at the 24th carbon atom.
The
vitamin D receptor AKA
VDR. According to the Wikipedia page [
WP] an alternative term for this is
calcitriol receptor.
I have never seen this term, but it is substantially more correct and
would ideally be widely used. However, I suspect that we are
stuck with the current terminology, and "VDR" is short and distinctive.
D3 and 25-hydroxyvitamin D have a very low affinity for the VDR, so it
is generally wrong to think of it as a receptor for these
compounds. 1,25-dihydroxyvitamin D (calcitriol) has a far greater
affinity for the VDR, but the lower affinities should not be forgotten
in scenarios where there is little 1,25-dihydroxyvitamin but very high
levels of D3 and 25-hydroxyvitamin D.
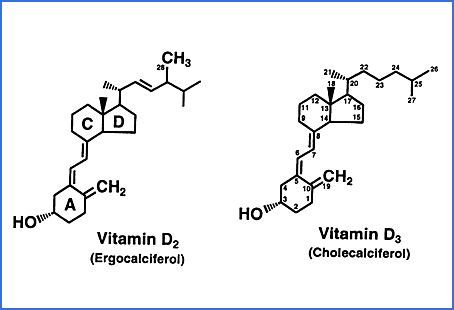
There are an alternative set of three compounds to those above, based on
Vitamin D2 ergocalciferol which
is produced industrially by UV-B irradiation of ergosterol, from fungi or yeast. This is not a normal part of mammalian
biology. Here is a rare diagram showing both the molecular
differences and the carbon numbers, from
this site.
(I am working on a Substack
article on the early vitamin D and I found there was a D1, of no
consequence, then D2 and D3. These are sequential numbers and do
not denote the carbon number to which a hydroxyl group is attached.
Both have a hydroxyl group attached to carbon 3. This is not
counted in the terminology in which the first normal biological
hydroxylation in the main vitamin D pathway of either compound is
regarded as the hydroxyl group attached to carbon 25.)
Vitamin D2 and its 25- and 1,25 hydroxylated forms have no
advantages over the
D3 compounds, so generally I don't discuss them. For some obscure
historical reasons doctors in the USA were only able to prescribe D2,
and often still do - I have been told that this is no longer
mandated. The definitive article on the D3 vs. D2 seems to be:
The term
vitamin is questionable. From a
book chapter cited
here , where the milk (at least in the USA in the 2020s) may be fortified with D2.
Although it is classified as a vitamin because of the nature of the
discovery process, vitamin D in actual fact should not be considered a
true vitamin for the following reasons. First and foremost, vitamin D
utilized by higher organisms can be formed in the epidermis of skin by
photolysis of 7-dehydrocholesterol, an intermediate in cholesterol biosynthesis.
Second, vitamin D is nearly absent from the food supply. Vitamin D is
found in fish liver oils, some fatty fish, and in egg yolk but is not
found in virtually all plant materials, in skeletal meats, seeds,
fruits, and vegetables. In fact, very little is found in an expected
source, milk. We consider milk an important supply of vitamin D in many
countries, primarily because it is fortified by the addition of vitamin
D.
It is common to find the phrase "vitamin D is a hormone" (
Google Scholar 711 hits)
as if this is technically more accurate than the questionable term
"vitamin" - or as if this important group of compounds deserves
additional gravitas not conferred by "vitamin". For additional
oomph, there is also "Vitamin D is a secosteroid hormone" (
328 hits).
Secosteroid refers to breaking the B ring of a steroid molecule [WP]
which is the only way known in nature and industry to make the vitamin
D compounds - and this break can only be achieved with a very narrow
set of wavelengths in the UV-B spectrum. It so happens that the
Sun produces these, in the top fraction of a percent of its frequency
range (the short wavelength end of its spectrum) and that if the Sun is
high enough in the sky, appreciable amounts of these wavelengths pass
through the atmosphere to reach the Earth's surface. The exact
wavelengths are a matter of research and debate, but are in the 293 to
297 nanometre range. See Industrial Aspects of Vitamin D Arnold L. Hirsch, 2011: https://sci-hub.se/10.1016/B978-0-12-381978-9.10006-X .
It is common for the term
vitamin D
to be used both for referring collectively to the compounds listed
above, and potentially other related compounds, which I think is a
correct
use of the term.
"Vitamin D" is also often used it when the author is actually referring to
one of these compounds, but does not refer to it specifically.
The reader is left to infer exactly which compound is meant, which is a
terrible
mistake in this already difficult field.
#2004-viethI
was very happy to find a 2004 article concerning common terminological
mistakes. This is from Prof. Reinhold Vieth, who has researched
vitamin D since 1975, and is still active in 2022. Google Scholar
reports
325 articles.
"Deltanoid" seems to refer to
analogues of "vitamin D" - non-natural molecules which mimic the
behaviour of naturally occurring vitamin D compounds. A company
of this name worked on these, but the term is obscure and probably not
used in 2021.
Abstract:
Official nutrition committee reports in both North America and Europe
now state that Vitamin D is more of a hormone than a nutrient.
These statements are wrong, and do not reflect the definitions of either
vitamin or hormone. Researchers often compound the problem by referring
to calcitriol or other deltanoids as "Vitamin D". These things have
serious consequences:
(1) The literature is burdened by an ongoing confusion that presumes that the reader will somehow “know” what the writer refers to by "Vitamin D".
(2) Medical practitioners not familiar with the ambiguities administer Vitamin D inappropriately when calcitriol or a deltanoid analog would be correct, or vice versa.
(3) Attempts to promote Vitamin D nutrition are hindered by alarmist responses justifiably associated with the widespread administration of any hormone.
Vitamin D is a vitamin in the truest sense of the word, because
"insufficient amounts in the diet may cause deficiency diseases". The
term prohormone is not
relevant to the Vitamin D system, but 25-hydroxy-Vitamin D (calcidiol)
is appropriately described as a prehormone, i.e. a glandular secretory
product, having little or no inherent biologic potency, that is
converted peripherally to an active hormone.
But even here there are
problems, since with sufficient UV-B skin exposure, there is no need
for D3 in food. In 2004 it was reasonable to think of
1,25-dihydroxyvitamin D as a "hormone" (the last word of the abstract)
because it was not widely known that it also has numerous non-hormonal
roles in intracrine / paracrine signaling.
As best I can tell, the term
hormone [
WP] originally meant
signaling molecule with the unstated assumption that the signaling path was from one type
of cell (or at least an organ somewhere) to one or more other cell
types (such as in one or more other organs), via the bloodstream (in
animals) or by fluids which are transported within plants.
This
signaling is the
conveyance of
information by way of the level (concentration) of the
compound. This is detected by the recipient cells.
In plants (which lack organs) the long distance, within the plant
itself, transport of the hormone molecules is by the plant's fluid
transport system. In vertebrates, the signal is
conveyed by the
level (concentration) of the substance in the bloodstream and
potentially the CSF (Cerebrospinal Fluid [
WP])
and interstitial fluid [
WP].
Unfortunately the term
hormone
is sometimes also applied to any signaling molecule - including those
which operate over short distances such as within cells and to nearby
cells, without involving the bloodstream at al.
Today, the only proper use of the term
hormone
in vertebrates is as described
above, and not to refer to signaling
molecules which operate within cells or between nearby cells.
Endocrine signaling [
WP]
is hormonal signaling for vertebrates. The Wikipedia page
concentrates on this, but also includes in the field of endocrinology
three other signaling modalities, while specifically excluding
neurotransmitter signaling: autocrine, paracrine and juxtacrine.
However, I am not at all sure that endocrinologists in general, or
those who write endocrinology textbooks, are really interested in these
fields, since they have nothing in common with hormonal signaling.
Juxtacrine signaling [WP]
involves cells in direct contact, including with junctions. This
is somewhat like synaptic communication with neurotransmitters, but is
not related to the nervous system. The vitamin D compounds are
not involved in this.
Here are the number of Google search hits within the English Wikipedia for:
autocrine 532
paracrine 659
intracrine 206
These are my notes on Hector F DeLuca's 2014
History of the discovery of vitamin D and its metabolites PMC3899558:
What we now know as D3 cholecalciferol
was isolated and its structure determined in 1937. Until the
1960s (I recall) the only way of assaying a substance for its D3
content was to feed varying amounts of it to baby rats (who were fed a
special diet which induces rickets in the absences of a certain amount
of vitamin D in the diet, or created by UV-B in their skin), to see
which
ones developed rickets. From this, the IU (International Unit)
was developed. This was approximatley the amount the baby rat
needed per day to avoid, or at least substantially reduce,
rickets. (I am working on a history of the vitamin D IU.)
This was later found to be 1/40,000,000 of a gram.
The impressively named International Unit is a very small quantity
indeed, and average daily D3 needs such as 1/8000 gram 5000IU appear to
many people to be scarily large. This article mentions autocrine
and paracrine, but not intracrine, signaling.
25-hydroxyvitamin D was discovered in 1968 and 1,25-dihydroxyvitamin D in 1971.
Here is my current (2022-05-14) understanding of the terminology. The Wikipedia references are for
general information and an indication of current usage, not to indicate
that these definitions are authoritative. I am not sure that
there is a single source of authoritative definitions of scientific
terms such as these, since usage and definitions change as research
progresses.
Intracrine signaling,
in which the compound acts as an "intracrine agent" or "intracine",
involves the intracrine agent being synthesised in the cell and being
sensed by a receptor
inside the same cell. This term is currently actively used, such as in this
2020 article of which
Professor Martin Hewison is one of the co-authors. He and his colleagues led the research into auto/intra/paracrine signaling in the late 2000s.
The Wikipedia page
https://en.wikipedia.org/wiki/Intracrine refers to these compounds as
hormones which is incompatible with the long distance signaling definition of "hormone" mentioned above. A Google search for
intracrinology reveals references going back to 1991
Intracrinology by Fernand Labrie
pubmed/1838082/ sci-hub , which contains the following diagram.
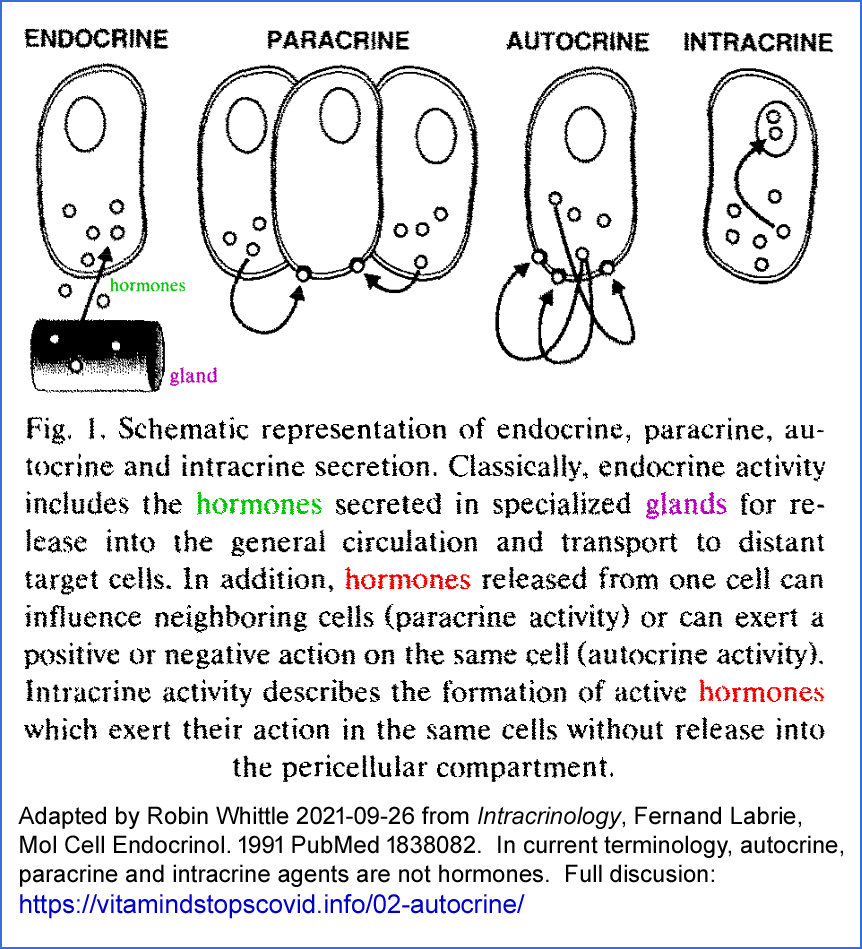
The Wikipedia page
https://en.wikipedia.org/wiki/Autocrine_signaling refers to the "hormone or chemical messenger" binding to receptors
on the cell which produced it. This implies outward facing receptors on the cell membrane.
Autocrine signaling, by the above WP page and the above diagram's definition,
involves a cell producing a compound which leaves the cell and then
activates receptors located in the same cell's membrane, from the
outside of the cell. There is no such
pattern with vitamin D compounds, where the signaling agent would be
1,25-dihydroxyvitamin D, since the VDR is an intracellular
receptor and is not found bound to or embedded within membranes.
In this definition, what would stop this process also causing the
activation of nearby cells of the same and or different type?
That would be paracrine signaling.
Autocrine signaling, by a broader, incorrect, definition,
which ignores the location of the receptor, includes both intracrine
signaling (above) and (non-existent for vitamin D compounds) narrowly
defined autocrine signaling. This means, that for vitamin D
compounds this use of "autocrine" signaling is a synonym for paracrine
signaling.
I guess this has happened for reasons including people not being fussed
about exactly where the receptor is located. The signaling system
is intracellular - from one set of events in the cell to cause another
set of events in the same cell.
Paracrine signaling [
WP]
broadly means a paracrine agent being generated in a cell of type X and
diffusing to nearby cells where it alters the behavior of other cells,
of type Y and/or perhaps of type X. The above diagram defines
this as involving receptors facing outwards from the cell membrane, but
I think the term also applies to the paracrine agent diffusing into the
recipient cell, where it binds to receptors in the cytosol. I
assume this is the case for 1,25-dihydroxyvitamin D paracrine
signaling, since VDR molecules are not located on the cell
membrane.
Potentially important loose end:
I have always assumed that cells whose
behaviour is affected by the very low level of hormonal
1,25-dihydroxyvitamin D detect the 1,25-dihydroxyvitamin D after they
diffuse into the cell, where they bind with VDR molecules. As far
as I know, most other vitamin D researchers assume this too.
However, here is an article which concerns a mechanism by which these
cells - all involved in calcium-phosphate-bone metabolism - actually
detect hormonal 1,25-dihydroxyvitamin D at the
outside of their cell membrane, perhaps
without using the VDR molecule at all.
Rapid Nontranscriptional Effects of Calcifediol and Calcitriol
Simone Donati, Gaia Palmini, Cinzia Aurilia, Irene Falsetti, Francesca Miglietta, Teresa Iantomasi and Maria Luisa Brandi
Nutrients 2022-03-14
https://www.mdpi.com/2072-6643/14/6/1291
|
I only glanced at the article and can't attest to its veracity.
When I read it and compare notes about it with some vitamin D
researchers, I will write more here.
#chauss-1
This is quite messy. The preprint of arguably the most important article ever written on the etiology of severe COVID-19:
uses
autocrine in the title,
but refers to the vitamin D based autocrine signaling as also
potentially involving paracrine signaling. The molecular
mechanisms all involve VDR in the cell, which according to the 1991
Labrie diagram, is
intracrine signaling. There is no mention of
intracrine. My summary of this dense cell-biology preprint is at:
https://aminotheory.com/cv19/icu/#2021-Chauss .
"Autocrine" also features in the title of the final article:
Autocrine vitamin D signaling switches off pro-inflammatory programs of Th1 cells
Daniel Chauss, Tilo
Freiwald, Reuben McGregor, Bingyu Yan, Luopin Wang, Estefania
Nova-Lamperti, Dhaneshwar Kumar, Zonghao Zhang, Heather Teague, Erin E.
West, Kevin M. Vannella1, Marcos J. Ramos-Benitez, Jack Bibby, Audrey
Kelly1, Amna Malik1, Alexandra F. Freeman, Daniella M. Schwartz, Didier
Portilla1, Daniel S. Chertow, Susan John, Paul Lavender, Claudia
Kemper, Giovanna Lombardi, Nehal N. Mehta, Nichola Cooper1, Michail S.
Lionakis, Arian Laurence, Majid Kazemian and Behdad Afzali
Nature Immunology 2021-11-11
https://www.nature.com/articles/s41590-021-01080-3 |
It is difficult enough getting doctors,
immunologists etc. to understand vitamin D's role in the immune system
without these terminological complications. Two recent immunology
textbooks
Janeways 9th 2017 and
Abbas
10th 2021 total 1500 pages and do not mention vitamin D in their
indexes. The only mention of autocrine and paracrine signaling is
in Janeways 9th, regarding cytokines in which
autocrine is defined as
affecting the behaviour of the cells which release the cytokine,
without reference to the location of the receptor.
Knowledge of vitamin D intracrine signaling has been developed
since the
mid-2000s. As far as I know, there are no accurate estimates of
how
many types of cell use vitamin D intracrine or intracrine-paracrine
signaling. See
#cells below.
For more in-depth material, a good place to start might be
articles which cite a 2010 article,
Autocrine and Paracrine Actions of Vitamin D by Howard A Morris and Paul H Anderson. Also:
Vitamin D metabolism and signaling in the immune system (
2012),
Vitamin D and immune function: autocrine, paracrine or endocrine (
2013) and
Vitamin D and immune function (
2013). These processes were not always described as "intracrine" or "paracrine", but these are the proper terms to use now.
As far as I know, there is no research article which presents
vitamin D based intracrine and/or paracrine signaling in an
easy-to-understand manner. So I created this web page.
Here are two other terminological and conceptual failings frequently found in vitamin D research articles:
-
Many MDs and researchers have no idea what intracrine (to some, autocrine) and
paracrine signaling are. They think that vitamin D (the
collective definition, or at least 1,25(OH)2D) "modulates" immune cells'
behavior solely by way of the 1,25(OH)2D produced in the kidneys and which circulates in the bloodstream as a hormone.
This is absolutely not the case, and it is a very serious
misconception. See, for instance, Figure 1 of Newmark et al.
2017 https://www.frontiersin.org/articles/10.3389/fimmu.2017.00062/full
This is an interesting article (I could follow it to about page 6)
about the evolution of the vitamin D systems. However, the
diagram showing 1,25(OH)2D going from the kidneys to the immune cells is just plain wrong.
They surely do reach these cells and probably diffuse into the cytosol,
after which they probably do bind with a VDR molecule. However,
the level of this1,25(OH) 2D is very low, and is very
stable and tightly controlled for the purpose of regulating
calcium-phosphate-bone metabolism. So how could it signal
information of use to immune cells? Furthermore, its level is
much lower than the localised levels of1,25(OH) 2D produced by intracrine and presumably paracrine signaling. See #02-nothorm below.
- It is common to write of people being vitamin D sufficient,
insufficient and deficient,
as if there is consensus on what this
means. Generally it means:
Sufficient: Greater than 30 ng/mL 75 nmol/L.
Insufficient: Between 20 and 30 ng/mL 50 and 75 nmol/L.
Deficient: Less than 20 ng/mL 50 nmol/L.
The question of healthy 25(OH)D levels is a
matter of debate - and it can't be assumed that there is a single
healthy level for all people. Maybe some individuals need more,
for instance people suffering from multiple sclerosis, rheumatoid
arthritis, psoriasis, cluster headache and migraine https://vitamindstopscovid.info/06-adv/. Perhaps 25(OH)D levels are only part of what constitutes good
health and other factors, such as Vitamin D Binding Protein (VDBP [WP])
characteristics and concentrations really matter too.
Ordinary blood tests are for total circulating 25(OH)D, and most of it is
bound tightly to VDBP or loosely to albumin, which reduce its
availability for diffusion to many cell types. See: #vdbp.
Researchers should report the proportion of people whose 25(OH)D levels
are below 30 ng/mL and above 20 ng/mL or whatever, not just that they
"are vitamin D insufficient".
#01-compounds
D3, 25(OH)D and 1,25(OH)2D - the three main vitamin D compounds
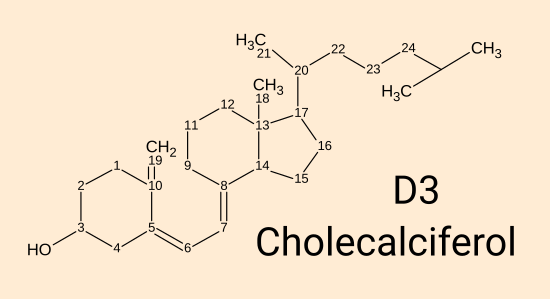
Vitamin D3 cholecalciferol. [
WP]
This is produced by 295 to 297 nanometre wavelength UV-B light acting on 7-dehydrocholesterol in the
skin. It can also be ingested in food or supplements. While this plain D3 directly
protects the endothelial cells which line our blood vessels [
Gibson et al. 2015],
all its other currently known roles in the body rely on it being
converted in the liver (there may also be some conversion in cells out side the liver), over a period of days to a week, by the
enzyme vitamin D 25-hydroxylase (encoded by the
CYP2R1
gene, a name sometimes given to the enzyme itself) to 25(OH)D.
(Another enzyme encoded by the CYP27A1 gene does the same thing and so
produces some of the 25(OH)D.)
The numbers indicate carbon positions. Most hydrogen atoms
are
not shown. The special trick to producing this from
7-dehydrocholesterol is to use 295 to 297 nanometre UV-B light to break
the second carbon ring between carbon 9 and 10. The resulting
molecule is not quite the shape of D3, but thermal motion over a period
of minutes or hours into the correct shape.
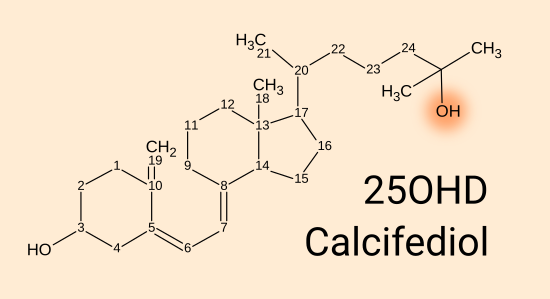
25(OH)D calcifediol = 25 hydroxyvitamin D3 = calcidiol [
WP].
This has an OH oxygen-hydrogen hydroxyl group at the 25 position, in
place of the H (not shown) which was there in D3. 25(OH)D circulates in
the blood, mainly
bound strongly to the vitamin D carrier protein and more weakly to albumin proteins. It is also
absorbed into fatty tissue, as is D3. Vitamin D blood tests
measure the total bound and unbound level of 25(OH)D. This level is
the most important part of the whole vitamin D system, however,
depending the amount which is unbound to the vitamin D carrier
protein and perhaps the albumin proteins may affect the amount of 25(OH)D
which diffuses from the bloodstream into the interstitial fluid between
the cells, and through their cell membranes into the cytosol of the
cells. .
Neither D3 nor 25(OH)D bind strongly to the vitamin D receptor [
W] which is a complex protein far bigger than these molecules.
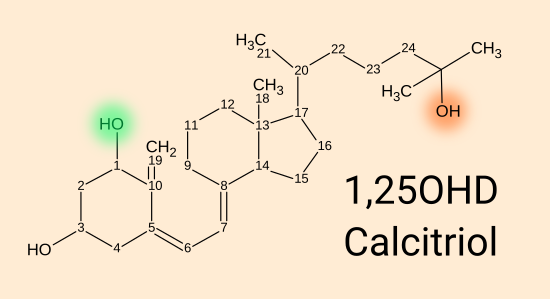
1,25(OH)2D calcitriol [
WP] = 1,25 dihydroxy vitamin D = 1,25(OH)
2 vitamin D, is produced by a
second enzyme,
1-hydroxylase, encoded by the
CYP27B1
gene, which adds an OH hydroxyl group at the 1 position to 25(OH)D.
This happens in the kidneys and inside many types of cells, including
immune cells.
1,25(OH)
2D binds strongly to, and so activates, the vitamin D receptor.
There is another enzyme CYP24A1 which
can add an OH hydroxyl group to the 24 position of 25(OH)D and 1,25(OH)
2D,
which is an
irreversible process. The resulting molecules are degraded and
excreted. The activity of this enzyme scales up with increasing
circulating 25(OH)D levels, and so gives rise to a strong self-limiting
process which reduces high 25OHD levels. This accounts for the curves in
the 25(OH)D by bodyweight and D3 intake graph from Ekwaru et al. 2014 at
01-supp/a-ratios/
. This
self-regulation makes it very hard to attain potentially toxic 25OHD
levels. Above 150 ng/mL (375 nmol/L) there is a risk of
hypercalcemia [
WP].
There is quite a lot of research into this self-imitating
system. I have not tried to understand the details and I
don't know to what extent there is consensus on how it
works, or even whether any researchers have reliably established the
mechanisms. This is a very important process and ideally I would
be able to understand and explain it better.
#02-nothorm
Intracrine and paracrine signaling with
25-hydroxyvitamin D and 1,25-dihydroxyvitamin D differs from
1,25-dihydroxyvitamin D as an endocrine signaling agent (hormone) and
is not significantly affected by this very low, stable, level of
circulating (hormonal) 1,25-dihyroxyvitamin D
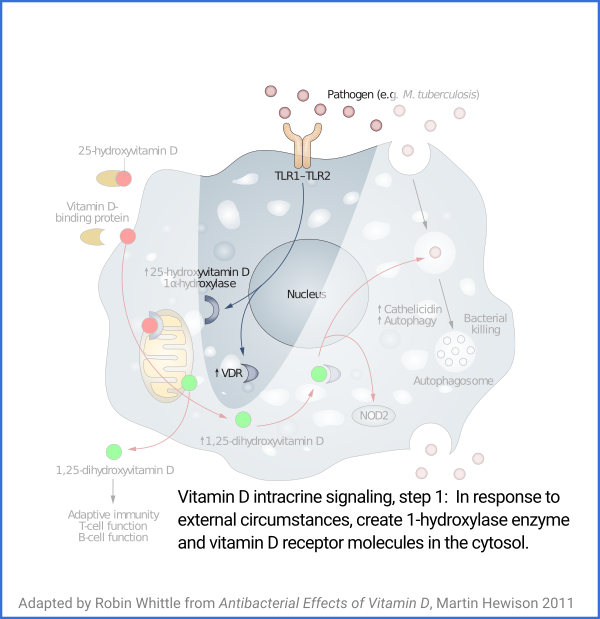
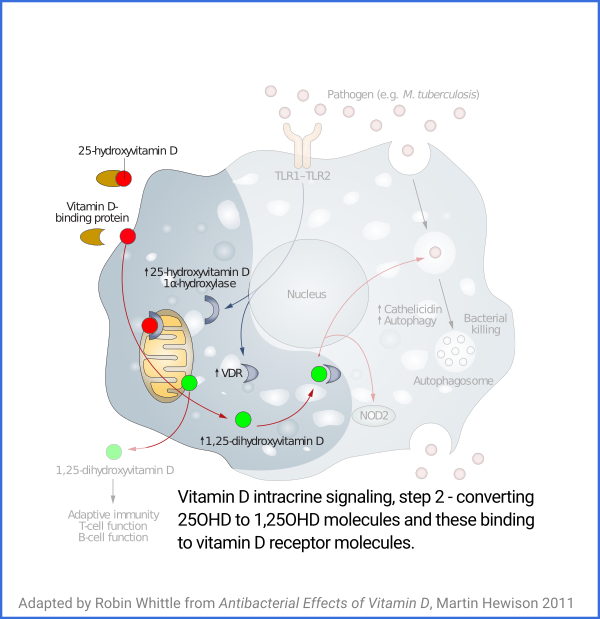
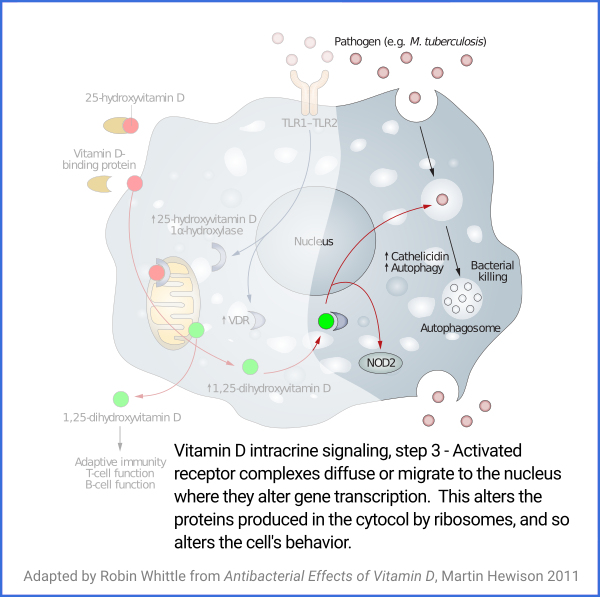
Vitamin D based intracrine signaling and paracrine signaling, at
least in immune cells, is not a continual process. The signaling
system is activated in a particular cell when certain conditions are
detected. As described in a section below
#05-intra, this causes both VDR (vitamin D receptor) and the
1-hydroxylase enzyme to be produced in the cytosol, whereupon the
enzyme converts the 25(OH)D which should already be there (and be
supplied from outside the cell, as it is consumed) into 1,25(OH)
2D, which
immediately binds to a VDR molecule. The bound complexes find
their way to the nucleus where they alter gene transcription, typically
upregulating and downregulating the transcription of dozens or hundreds of
genes.
Vitamin D based intracrine/paracrine signaling is the same general
system in multiple cell types. Tens or hundreds of millions of
years of evolution have used these systems for a variety of purposes -
a different purpose in each type of cell - with the activating
conditions and the changed cell behaviour being entirely cell-type
specific. So there is no generalised way of describing these
signaling systems in terms of what activates them and what changes they
create in cell behaviour - since these vary widely from one cell type
to the next.
In this section I explore something I haven not seen explicitly
tackled
in the research literature, though the common misconception of "vitamin
D" being a hormone was tackled by Reinhold Vieth in 2004, as mentioned
above:
#2004-vieth :
The question is:
To what extent, if any, are intracrine signaling
operations in immune cells affected by the relatively stable, and very
low, level of hormonal 1,25(OH)2D in the bloodstream?
As far as I
can see, this hormonal level is too low to significantly affect
intracrine signaling, so moderate changes in that hormonal level (within
whatever range it might healthily change, in order to maintain
the correct balance between calcium absorption, excretion and blood
levels) would
also have no significant effect on individual immune cells or on the
whole immune system.
A related question is the same regarding
paracrine signaling. I
don't know of any measurements of the levels of 1,25(OH)
2D diffusing to
nearby cells, but these levels will be somewhat or perhaps a lot lower
than the levels at which 1,25(OH)
2D is generated intracellularly.
For paracrine signaling to work at all, it needs to be sensitive to
these diffused levels, and not significantly affected by low levels of hormonal 1,25(OH)
2D
which presumably diffuse from the
bloodstream into the interstitial fluid between the
cells, and from there diffuses across the cell membrane into the
cytosol of the cell. Below, I attempt to answer this question
quantitatively. It seems likely that paracrine signaling involves
diffused levels
of 1,25(OH)
2D which are well above the hormonal level of 1,25(OH)
2D.
I don't have references handy for this, but I recall that some or many cells
have a 24-hydroxylase enzyme which irreversibly degrades 25(OH)D, 1,25(OH)
2D
and think D3. It would make sense for this to be active, to
some probably small degree, in cells which are involved in vitamin D based intracrine /
paracrine signaling since these are time-sensitive, rather than
long-term, relatively static, processes. For the gene
transcription changes, which occur when the intracrine / paracrine
system is operating, to be reverted back to normal patterns of gene
transcription, it would make sense for the cell to have a certain
degree of 24-hydroxylase enzyme, to mop up 1,25(OH)
2D rather than let it
float around for tens of hours or more. (I guess the activated
complexes of the 1,25(OH)
2D bound to the VDR are degraded in due course,
for the same reason.) To the extent that there is a
non-trivial level of 24-hydroxylase enzyme activity in the cell, it is
reasonable to think that this would be continually degrading any
hormonal 1,25(OH)
2D which diffused into the cell.
Here is a description of
the one
hormonal function of the vitamin D compounds. All doctors understand this mechanism.
A hormone is a
compound in blood circulation (or perhaps the cerebrospinal fluid), whose level (concentration) is
controlled, with that level affecting the behaviour of one or more cell
types which could be anywhere in the body.
Carefully regulated, very low, levels of 1,25(OH)2D are produced in the kidney from
25(OH)D and are put into circulation in the blood as a
hormone to regulate calcium-phosphate-bone
metabolism.
This hormonal 1,25(OH)
2D has a
half life of around 6 hours, which is much
shorter than the month or so half life of 25(OH)D (or shorter with higher
concentrations and longer if the levels are very low, such as below
20 ng/mL) .
In one
study, 25(OH)D levels averaged
36 ng/mL
(91 nmol/L = 36 parts per billion by mass), which is around twice what
people attain without much high elevation direct
sunlight skin exposure or proper vitamin D3 supplements. (There is
little D3 in food or multivitamins, and the UK's 0.01mg 400IU a day is
a scandalously small amount - a total of 0.29 grams, the mass of 18
grains of jasmine rice, if this amount was taken for 80 years.)
With 259OH)D levels above, very
approximately, 20 ng/mL (maybe more is needed for some people, such as
those over 50) the kidneys convert enough
circulating 25(OH)D into 1,25(OH)
2D to maintain whatever level of
circulating
(hormonal) 1,25(OH)
2D
is needed throughout the body to maintain proper
calcium levels in the blood. The calcium level (calcium ions, in
solution in the blood plasma), is sensed by the parathyroid glands
to the control the parathyroid hormone level, which controls the
kidneys'
rate of hydroxylating 25(OH)D to 1,25(OH)
2D.
In this study, the average circulating (hormonal) 1,25(OH)
2D level was
0.045 ng/mL (45 parts per trillion, 0.111 nmol/L, which is
1/800th of the 36 ng/mL 25(OH)D level.
So every 6 hours the kidneys convert about 1/1600th of the circulating
25(OH)D into circulating 1,25(OH)
2D.
Assuming a 100% conversion rate (and for D3 to 25(OH)D, the efficiency
is, very approximately, 25%), over a month, this requires about
1/12th of the circulating 25(OH)D. Since the half-life of this
circulating 25OHD is a month or so, we can guesstimate that
about 1/6th
of 25OHD lost every month is due to conversion in the kidneys to
hormonal 1,25(OH)2D.
The other 5/6th of the loss of 25(OH)D must be due to its
use in intracrine and paracrine signaling
in many cell types
all over the body, and to the 25(OH)D being degraded by the
24-hydroxylase enzyme. (In states of intense disease, the immune
system may consume more than its usual amount of 25(OH)D, so the half
life would be shorter.)
25(OH)D levels of
10 ng/mL 25 nmol/L or less in children causes rickets [
WP]
- failure of the bones to grow strong and straight. This is due
primarily to the kidneys being unable to maintain a suitable level of
hormonal 1,25(OH)
2D, but would also be due, in part, to excessive
inflammation and other immune system failures due to immune cells
having ~~1/5th of the 25(OH)D they need for their intracrine and paracrine
signaling systems to work properly.
So at healthy 25(OH)D levels such as
50 to 80 ng/mL 125 to 200 nmol/L,
we can assume that (very approximately) only a tenth or less of the
25(OH)D produced from D3 is used by the kidneys for the one hormonal
function of the vitamin D compounds.
While 1,25(OH)
2D (discovered in 1972) is the best known has a
hormone for its role circulating in the blood, its production (in the
kidneys, as just described) is not where most of the 25(OH)D (produced from D3)
is used. Until about 1979, kidney conversion was the only known
source of 1,25OHD. Then, extra-renal (outside the kidneys)
conversion
to 1,25(OH)
2D was first discovered (
Gray et al. 1979). In 2007 an important article was published, discussing vitamin D intracrine and paracrine signaling:
#1ng
These researchers used some macrophages and monocyte derived dendritic
cells, both with their intracrine / paracrine signaling systems turned on,
to find out how their conversion of 25(OH)D to 1,25(OH)
2D was affected by
differing levels of 25(OH)D: 2, 20 and
60 ng/mL.
(I
am not sure that this is directly equivalent to such levels in the
bloodstream plasma, in vivo, where the 25(OH)D is mainly strongly bound
to the circulating vitamin D binding protein, and to e lesser extent
to albumin proteins, with only a small fraction available for diffusion
into tissues and/or immune cells.) The levels of 1,25(OH)
2D produced, after 48 hours, were (Fig 1 levels divided by 2.5 to
give ng/mL) approximately 0.013, 0.12 and
1 ng/mL respectively.
The 0.12 ng/mL 1,25(OH)
2D (resulting from 20 ng/mL 25(OH)D supply to the
cells) only marginally affected the gene transcription and protein
synthesis which intracrine signaling in the macrophages drives.
This is upregulation of CD14 [
WP] and downregulation of three other proteins (Fig. 2). The
1 ng/mL 1,25(OH)
2D level, produced when
60 ng/mL
25OHD was supplied to the macrophages) fully upregulated CD4 and
downregulated the other three proteins - the effect was just as strong
as when 40 ng/mL 1,25(OH)
2D was added to the cells.
Some important points arise from the abovementioned research:
- Researchers in 2007 determined that 25(OH)D levels at the cells needed to be more like 60 ng/mL than 20 ng/mL for
intracrine signaling to work properly. 25(OH)D is consumed in
those cells by intracrine / paracrine conversion to 1,25(OH)2D - at the times when this signaling system is activated - and
slowly, at all times, by 24-hydroxylase which consumes a little of it,
degrading 25(OH)D to 24,25(OH)2D. Since 25(OH)D diffuses to
all cells from the bloodstream, it follows that
blood 25(OH)D levels need to be more like 60 ng/mL than 20 ng/mL for good health.
#vdbp
As far as I know, the exact concentrations of 25(OH)D inside cells such
as immune cells is not known. There is some uncertainty or debate
about how 25(OH)D molecules get from the bloodstream (the plasma,
fluid, part of the blood, not from blood cells) to the interstitial
fluid and then into the cells, as discussed in the next cited
article. There is no evidence of active transport of 25(OH)D into
immune cells, though I recall reading that the kidney cells which need
25(OH)D transport it across their cell membranes with the 25(OH)D bound
to the much larger Vitamin D Binding Protein VDBP
molecule. Most of the 25(OH)D in the bloodstream is in these
bound complexes, so this implies a high-efficiently transport system
compared to relying on the much smaller amount of unbound 25(OH)D.
According to:
Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions
Daniel David Bikle and Janice Schwartz
Frontiers in Endocrinology, Bone Research 2019-05-28
https://www.frontiersin.org/articles/10.3389/fendo.2019.00317
85% of serum 25(OH)D is strongly bound to VDBP.
15% is more loosely bound to albumin proteins.
0.03% is unbound, freely in solution in the plasma.
There is a question of how, when the great majority of 25(OH)D in the
bloodstream is bound to VDBP or albumin proteins, can more than the
very small fraction of it which is in solution (unbound to any
molecule) diffuse into the interstitial fluid and so into cells, where the
final concentration in those cells must, as best we know, be no greater
than the concentration of unbound 25(OH)D in the bloodstream, which is
very low.
Fletcher et al. 2022 cited above #fletcher
states (pp 4, middle of right column), that reduced levels of VDBP with
a given total concentration of 25(OH)D enhanced the ability of that
25(OH)D to facilitate antibacterial responses by monocytes and
dendritic cells. This implies strongly that the cell's supply, by
passive diffusion, of 25(OH)D from the bloodstream depends largely or
wholly, for any given concentration of 25(OH)D on the proportion of
25(OH)D which is not bound to VDBP.
- Now, in 2020, we reasonably assume* that many or most types of immune cell need 25(OH)D, which they consume by hydroxylating it to 1,25(OH)2D when their intracrine / paracrine signaling systems are activated - and that an unknown
number of other cell types also need for the same reason. The
cells of the types which sense 1,25(OH)2D as a paracrine agent don't
necessarily produce it themselves. If they don't produce it, they
presumably don't need 25(OH)D, but the nearby cells, probably of
different types, which produce this paracrine 1,25(OH)2D do need 25(OH)D
- and they may well produce 1,25(OH)2D for their own use as an intracrine agent
as well.
#cells
* The Fletcher et al. 2022 article cited above #fletcher reports on Martin Hewison's team's research with macrophages [WP] and dendritic cells [DCs WP], both types of cell which are derived from monocytes [WP]. They mention (start of pp 5) that both macrophages and DCs may emit 1,25(OH)2D as a paracrine agent to affect the behavior of nearby T lymphocytes.[WP] and that cytotoxic T cells may also emit 1,25(OH)2D as a paracrine agent. They cite Chauss et al. 2021's #chauss-1
beautiful and extensive (my value judgment) research with intracrine
signaling in Th1 regulatory lymphocytes (though, as noted above, the
title of this article uses the term "autocrine"). I don't have a
reference handy, but the genes for both VDR and the 25-hydroxylase
enzyme are expressed in many types of immune cell and in other cell
types which likewise are not involved in calcium-phosphate-bone
metabolism. While researchers have
not delved into all these cell types regarding their vitamin D based
intracrine and paracrine signaling activity, it is reasonable to assume
that any cell types such as these express these genes for the purposes
of vitamin D based intracrine and/or paracrine signaling.
- Yet
still, despite increasingly desperate protests from some MDs and
researchers, in the midst of the COVID-19 pandemic, many government
official guidance documents on vitamin D3 supplementation are still based on
the outdated (and even at the time, mistaken) 2010 conclusions of the
US Institute of Medicine ../01-supp/#iom and https://nutritionmatters.substack.com/p/government-vitamin-d3-supplementation , which seek only to achieve 20 ng/mL as needed for bone health, with no regard to the higher levels needed for good immune system health.
Please remember these numbers. In other pages here (see https://vitamindstopscovid.info/00-evi/) you
can read arguments that if everyone supplemented vitamin D3 to attain, on
average, around 50 ng/mL 125 nmol/L
25(OH)D, that SARS-CoV-2 would only rarely cause severe symptoms, with
those infected shedding much fewer viruses on average, causing
transmission to be much lower than today - so there would be no COVID-19
pandemic, or at least not one to worry much about.
- The hormonal, circulating, 1,25(OH)2D level of around 0.045 ng/mL can
be expected to diffuse into cells which use 1,25(OH)2D as part of their intracrine signaling systems. (What level this occurs at in those
cells depends on how much of that serum, circulating, hormonal 1,25(OH)2D
is bound to vitamin D binding protein and albumin proteins - I haven't
read the details, but I guess only a fraction is free to diffuse into
the interstitial fluid and cells.)
- This is not enough to significantly
activate the gene transcription changes of the intracrine / paracrine
signaling systems, since they are only marginally activated by
0.12 ng/mL 1,25(OH)2D - and it takes about 1 ng/mL to fully activate them.
So while all these cells are bathed in hormonal 1,25(OH)2D, the level of this - which is generally stable - is too low to significantly affect their
intracrine or paracrine signaling systems.
#03-minlev
At least 50 ng/mL 25(OH)D blood levels required for good immune system function
The importance of proper (at least
50 ng/mL
= 125nmol/L = 1 part in 20 million by mass) levels of 25(OH)D is not
widely enough known. While lower
values may be sufficient for the kidneys to maintain the proper level of hormonal 1,25(OH)
2D, we need
at least this level of 25(OH)D for numerous types of cell - especially
immune cells - to function correctly. These cell types (as noted above
#cells,
of which only a few have been properly researched) need good supplies
of 25(OH)D for their their intracrine / paracrine signaling systems,
which play a likely major role in the ability of each individual cell
to respond to its changing circumstances .
Cannell et al. 2006 proposed that
50 ng/mL (125 nmol/L) be the target 25-hydroxyvitamin D level, all year round:
Epidemic influenza and vitamin
D
J. J. Cannell, R. Vieth, J. C. Umhau, M. F. Holick, W. B. Grant,
S, Madronich, C. F. Garland and E Giovannucci
Epidemiology & Infection 2006-09-07
https://www.cambridge.org/... |
The target range of
40 to 60 ng/mL (100 to 150 nmol/L) was stated in 2008 by 48 leading researchers and MDs in the Call to D*Action:
This approximately
50 ng/mL level was fully justified by the research of Quraishi et al. 2014, mentioned in a section below:
#04-quraishi
Yet the Institute of Medicine (IOM) chose
20 ng/mL
in 2011, in a major report, which forms the basis of most of today's
(2022) government recommendations regarding 25-hydroxyvitamin levels
and D3 supplementation quantities to attain this. This was
a huge mistake, including their botched calculation for how much D3
people should be taking.
See my article on this:
This 2020 review article, co-authored by the world's leading vitamin D researcher, (Prof. Michael Holick) also calls for
40 to 60 ng/mL 25-hydroxyvitamin D:
Immunologic Effects of Vitamin D on Human Health and Disease
Nipith Charoenngam, Michael F. Holick 2020-07-15
Nutrients 2020, 12(7), 2097
https://doi.org/10.3390/nu12072097
|
This article and another one:
Disassociation
of Vitamin D’s Calcemic Activity and Non-calcemic Genomic Activity and
Individual Responsiveness: A Randomized Controlled Double-Blind
Clinical Trial
Arash Shirvani, Tyler Arek Kalajian, Anjeli Song & Michael F. Holick, Nature Scientific Reports 2019-11-27
https://www.nature.com/articles/s41598-019-53864-1
|
report on hundreds of genes which are upregulated or downregulated
by
vitamin D in a sample of white blood cells. Below, I explain how
the upregulation occurs, but not the downregulation since I don't yet
understand the molecular mechanisms, which are very complex and involve
the exact way in which DNA is uncoiled and formed around histones, so
it is exposed to enzymes which copy its information into messenger RNA.
All these genes are affected as
part of intracrine / paracrine signaling in an unknown number of cell
types
#cells, including probably most or all immune cell types.
So there seems to be a large and so-far undefined number (I guess
dozens to hundreds) of cell types who respond to their circumstances in
part, at least, via vitamin D based intracrine / paracrine signaling.
This means vitamin D (the three compounds in general, but in the cells
themselves, just 25(OH)D and 1,25(OH)
2D) are extraordinarily important for
most or all systems of the body. The scope of vitamin D's role in
the body extends beyond the proteins for which these specific genes
provide the instructions, because some of these genes involve proteins
which affect histones [
WP]. Histones are proteins which
physically organise the long DNA molecules of the chromosomes, 1.8
metres in total. An important role of the histones is to unwind
particular regions of the DNA so its genes can be copied into messenger
RNA molecules and so direct the cell's protein making machinery.
To whatever extent vitamin D intracrine / paracrine signaling affects
histones, it therefore affects numerous other aspects of the cell's
ability to perform its functions.
Also, since in the case of Th1 regulatory lymphocytes at least
#chauss-1,
vitamin D based intracrine / paracrine signaling affects the production
of cytokines (both pro- and anti-inflammatory), the actions of other
cell types and in this case the destruction of pathogens and the body's
own cells are also affected by vitamin D based intracrine / paracrine
signaling.
40 to 60 ng/mL (100 to 150 nmol/L) was also suggested as the proper target range in this 2019 article (68
citations):
This article also discusses the benefits some people find from much
higher 25(OH)D levels, for suppressing inflammatory disorders such as
psoriasis and rheumatoid arthritis. Please see
https://vitamindstopscovid.info/06-adv/ for more on this and how it relates to our lack of helminths (intestinal worms).
Please also see the recent article from MDs in Dubai who had great
success with COVID-19 patients by either previously raising their 25(OH)D
levels to the
40 to 90 ng/mL 100 to 225 nmol/L
levels or by using the same bolus D3 and then body-weight ratio
continuing supplemental D3 intakes on newly diagnosed hospitalised
COVID-19 patients. The link and my summary is at:
https://aminotheory.com/cv19/#2020-Afshar .
Here is another recent research article:
Editorial – Vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections
A. Fabbri, M. Infante, C. Ricordi Eur Rev Med Pharmacol Sci 2020; 24 (7): 4048-4052 2020-04-05
https://www.europeanreview.org/article/20876
|
They mention that
40 to 60 ng/mL circulating 25OHD is
required for the autocrine signaling system of immune cells to function properly. The proper term for this is
intracrine signaling.
In this article's title, the term "modulator of . . . " is potentially
misleading, especially when it is used with the overly general term
"vitamin D". In-vitro addition of 1,25OHD (calcitriol) to
immune cell cultures will change their behaviours in ways which
resemble healthy responses, so it is reasonable to state this
experimental
addition of 1,25(OH)
2D "modulates" immune responses. However,
this does not resemble the natural process of intracrine / paracrine
signaling, in which 11,25(OH)
2D is locally (intracellularly or in a nearby
cell) produced only in particular circumstances.
Similarly, if an in-vitro cellular system or an in-vivo mammal's immune
system changes its behaviour upon the experimental addition of extra
25(OH)D, this does not mean that in Nature, the level of 25(OH)D modulates
anything, like a level of a hormone "modulates" some cells'
behaviour. All that is happening is that the immune cells or
immune system are functioning better than before, due to proper
supplies of 25(OH)D rather than baseline state of being unable to work
properly due to insufficient 25(OH)D for their intracrine / paracrine
signaling. (Also, if the level of 25(OH)D is excessive, the
functioning of the immune system may be degraded, but this is
over-supply, exceeding the proper operating conditions of that system,
not signaling anything as the level of a hormone does.
The text (in the quote below) "
the beginning point of the plateau where the synthesis of the active form calcitriol becomes substrate-independent"
requires some explanation for non-specialists. The 1-hydroxylase [
WP] enzyme (molecular diagram below) is a
large, complex protein, whose actions are powered by some other
molecules which are changed in the process. The authors are
discussing the hydroxylation of 25(OH)D to 1,25(OH)
2D, which is a crucial early
step in intracrine signaling. There are multiple 1-hydroxylase enzyme
molecules in the cell, and each converts one 25(OH)D molecule at a time
to 1,25(OH)
2D. The speed of this conversion is important,
since if it is too slow, then the 1,25(OH)
2D levels in the cytosol (main
body of the cell, where this happens - not in the nucleus) will not
raise to a high enough concentration (as noted above
#1ng), around
1 ng/mL (1 part per billion by mass) that a sufficient number of these 1,25(OH)
2D molecules will bind with vitamin D receptor molecules, after
which some of these bound complexes will migrate (or at least diffuse) to
the nucleus, as I will describe
properly below.
There are also a few
24-hydroxylase enzyme molecules in the cell, converting any 1,25(OH)
2D
they find to inactive 1,24,25(OH)
3D which is broken down into compounds
which are excreted. (This enzyme does the same thing to the more
numerous 25(OH)D molecules in the cell: convert them to 24,25(OH)
2D which is
also broken down and its components taken out of the cell, ultimately to be excreted.)
This serves two
purposes. Firstly, mopping up any hormonal (from the bloodstream) 1,25(OH)
2D which diffused into the cell, to reduce the degree to which it
might activate the rest of the intracrine signaling system.
Secondly, to slowly mop up 1,25(OH)
2D previously produced by the intracrine
signaling system operating normally, so that the levels drop after this
system is no longer activated. In a further twist, some of
these
enzyme molecules are formed differently and don't convert 25(OH)D or 1,25(OH)
2D, they just bind to them for a while and so are described as
decoys. [
Cantorna et al. 2015, and also Hewison et al. 2007, above.]
If there is no 25(OH)D, the intracrine signaling system cannot
work. If there is too little, then it will work too slowly, or
not work properly - so the cell will not respond fully to its new
circumstances and our health will suffer.
The enzyme itself is not changed - it is a catalyst. When,
by random thermal motion, a molecule of 25(OH)D is in the right position
in the enzyme's active site, the enzyme replaces the hydrogen H which
is bound to the number 1 carbon C atom with an oxygen-hydrogen OH
hydroxyl group, after which. the newly-formed 1,25(OH)
2D is no longer so attracted to the enzyme's active site, and
floats
away.
The 25(OH)D molecule, up to the point where it is converted to 1,25(OH)
2D, is the
substrate.
The authors imagine a graph with 25(OH)D concentration being the
horizontal axis and the total rate of conversion to 1,25(OH)
2D being the
vertical. The
plateau
they refer to is where the rate of conversion no longer rises linearly
(upwards and to the right) with 25(OH)D concentration, due to the limiting factor being mainly the
enzyme's own intrinsic speed of conversion, when it has it hardly has to
wait for a fresh 25(OH)D molecule to arrive in its active
site.
We also believe that maintenance of circulating 25-hydroxyvitamin D levels of 40 - 60 ng/mL would be optimal, since it has been suggested that concentrations amounting to 40 ng/mL represent the beginning point of the plateau where the synthesis of the active form calcitriol becomes substrate-independent [2011-Hollis err] [2018-Wagner].
Additionally, serum 25-hydroxyvitamin D levels of approximately greater than or equal to 40 ng/mL
could provide protection against acute viral respiratory infections, as
demonstrated in a prospective cohort study published in PLoS One and
conducted on 198 healthy adults [2020-Sabetta]. To reach these concentrations in adults, a dietary and/or supplemental intake of vitamin D up to 6000 IU/day
– deemed to be safe – is required. However, elderly subjects,
overweight/obese and diabetic patients, patients with malabsorption
syndromes, and patients on medications affecting vitamin D metabolism may require even higher doses under medical supervision.
|
The authors mean that if 25(OH)D levels (in the blood) are around
40 ng/mL
or more, then this leads, via diffusion - there being no
active transport of 25(OH)D from the bloodstream into the fluid between
the cells and across the cell's membrane - to a concentration of 25(OH)D
in the cell to start with which, after the cell's intracrine signaling
system is activated (by the creation of 1-hydroxylase enzyme and VDR
molecules in the cytosol) which enables each such 1-hydroxylase enzyme
molecule to work at close to
its full speed hydroxylating these 25(OH)D molecules to 1,25(OH)
2D.
Also, this 25(OH)D level in the blood is required to maintain the
25(OH)D
levels in the cell as some of the 25(OH)D is consumed by the conversion
process. So this 40 ng/mL or so level in the bloodstream is
required so that passive diffusion (probably from the relatively small
proportion (15%) of 25(OH)D which is not bound to the vitamin D binding
protein
#vdbp)
results in enough 25(OH)D diffusing
into the cells as the intracrine hydroxylation process continues, so
the
enzyme is not slowed down by too low a level of 25(OH)D in the
cell. If there was too low a level of 25(OH)D in the cell, each
enzyme molecule would need to wait, on average, an
excessive amount of time before a fresh
25(OH)D molecule to arrived at its active site in the correct
orientation.
The key thing to remember is that 25(OH)D levels are very low. A healthy level is
50 ng/mL, (as explained in the next section
#04-quraishi) but many people, without supplements, never achieve this. So for many
people, average levels are 1/2 or even as low as 1/10th of this.
50 ng/mL
(50 parts per billion) is only one part by mass of 25(OH)D per 20,000,000 parts by mass of all
the water and other compounds in the cell. So these are quite
rare molecules. A 70kg person only needs a gram of D3 every
22 years, about 1/3 to 1/4 of which is converted to 25(OH)D in the liver, to maintain
this healthy level. (50 parts per billion is like a 3.7mm cube of water in a cubic metre of water.)
Here I am assuming that the concentration of 25(OH)D in the cell is
about the same as that in the bloodstream, but as noted above
#vdbp
it is probably a lot lower than this, since it seems to derive, by
diffusion, from the 15% which is not tightly bound to the vitamin
D binding protein, almost all of which is more loosely bound to albumin
proteins.
#thermal
You probably began reading this page thinking of the COVID-19 crisis,
the influenza crisis and perhaps the
sepsis crisis. Now you are contemplating lonely 25(OH)D
molecules being jostled around by the thermal vibrations of surrounding
molecules (mainly water) until one of these molecules:
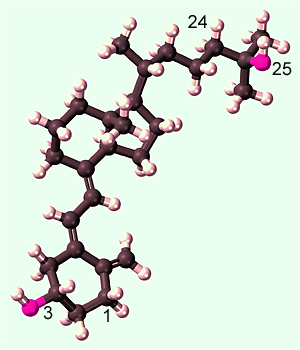
- Arrives very close to the active site of the much larger enzyme molecule. This is 3
dimensions (X, Y and Z) of movement over large distances (one such molecule on
average per ~320 nanometres cubed) compared to the size of the 25(OH)D
molecule (~0.2 nanometers) and the enzyme molecule:
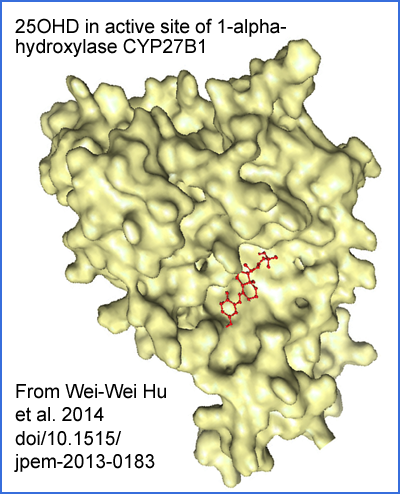
Adapted from paywalled article https://www.degruyter.com/document/doi/10.1515/jpem-2013-0183/ Sci-Hub: https://sci-hub.se/10.1515/jpem-2013-0183 Wei-Wei Hu et al. A novel compound mutation of CYP27B1 in a Chinese family with vitamin D-dependent rickets type 1A 2013-11-07 .
- Is pointing in exactly the right direction for it to fit. This needs to be correct in 3 rotational dimensions in order to align the end-to-end axis of the
molecule with the axis of its position in the enzyme's binding
site.
- Is rotated correctly along its axis - this is 1
axial rotational dimension - so the 25(OH)D molecule is precisely aligned
with the matching outer electron orbitals of the atoms of the enzyme's
binding site..
When this happens, the positive and negative charges (due to
some negatively charged outer electron orbitals being offset from the positively
charged nucleus they surround) on particular
parts of the two molecules will draw them closer, the 25(OH)D molecule will be
fully docked, and the enzyme and its co-factor molecules will do their
work of attaching the OH to the number 1 carbon atom.
This probably seems a long way from the immune system and COVID-19, but it is absolutely germane. If everyone in the world had
50 ng/mL or more 25(OH)D in their blood, then:
- The enzymes in all their cell types which use vitamin D for
intracrine / paracrine signaling would not be waiting long for another
25(OH)D molecule to dock in their active sites.
- So they would produce 1,25(OH)2D at a perfectly healthy rate whenever the cell's intracrine signaling system is activated.
- The intracrine signaling systems of all cell types (including many
types of immune cell) would work correctly, making then respond fully
and rapidly to their changing circumstances.
- Although
there are numerous other factors affecting total immune
system performance, this would mean that the current vitamin D
deficiency epidemic would not exist - and it is low vitamin D which is
the primary cause of some immune responses being weak, while others are
dysregulated - meaning overly-aggressive, hyper-inflammatory and
self-destructive. These weak and dysregulated immune
responses are the primary or sole reason why some people who are
infected with SARS-CoV-2 develop severe COVID-19. (The
dysregulated, hyper-inflammatory responses are also caused by lack of
helminths: https://vitamindstopscovid.info/06-adv/#02-helminths .)
- So
almost all people would fight off the SARS-CoV-2 infection
without serious symptoms. Likewise flu. Also, very few
people would develop sepsis, Kawasaki disease or Multisystem
Inflammatory Syndrome. Likewise pre-eclampsia, which is a dysregulated, hyper-inflammatory,
immune disorder of pregnancy.
(Note: there is a lot of interest in the idea that high vitamin D
levels will substantially reduce the chance of being infected with
COVID-19 for any given vital insult. I see no evidence that
this is more than a marginal effect. The most important point, for all society, is the
next one, followed by the just-mentioned great reduction in average
severity.)
- For those infected, average
total quantities of viral shedding would also be greatly reduced, so
fewer people would become infected. COVID-19 would not
spread very much at any time of year. Likewise flu.
- So there would be no COVID-19 crisis, with no need for lockdowns,
social distancing, vaccines or masks. The few who did become
seriously ill could be treated with oral 25(OH)D (calcifediol) and D3 ../04-calcifediol/
as well as other early treatment techniques: https://c19early.com.)
You now have an understanding of a crucial part of the current global crisis down to a
molecular level - and if you want to, you can look up the gory details
of the virus, the ACE2 receptor, the destruction of the endothelium,
the hypercoagulative state of the blood and the microembolisms and
larger clots in the lungs, brain, heart, spinal cord, liver kidney etc.
None of those gory details would matter as much as they do now, because
they would not exist to anything like the current extent,
if everyone had enough vitamin D. 70kg adults, on
average, to attain about
50 ng/mL (125 nmol/L) 25(OH)D, without relying on UV-B skin exposure, or the small amounts of D3 in food, need to ingest
45 milligrams of D3 year = a gram every 22 years. This is
0.125mg 5000 IU a day. Pharma grade D3 costs about
USD$2.50 a gram, ex-factory.
Please see
https://vitamindstopscovid.info/01-supp/
for D3 supplemental intakes, as ratios of bodyweight, which I derived
from the work of Ekwaru et al. 2014, and for an article by Iranian
doctors working in Dubai who found similar ratios worked really well.
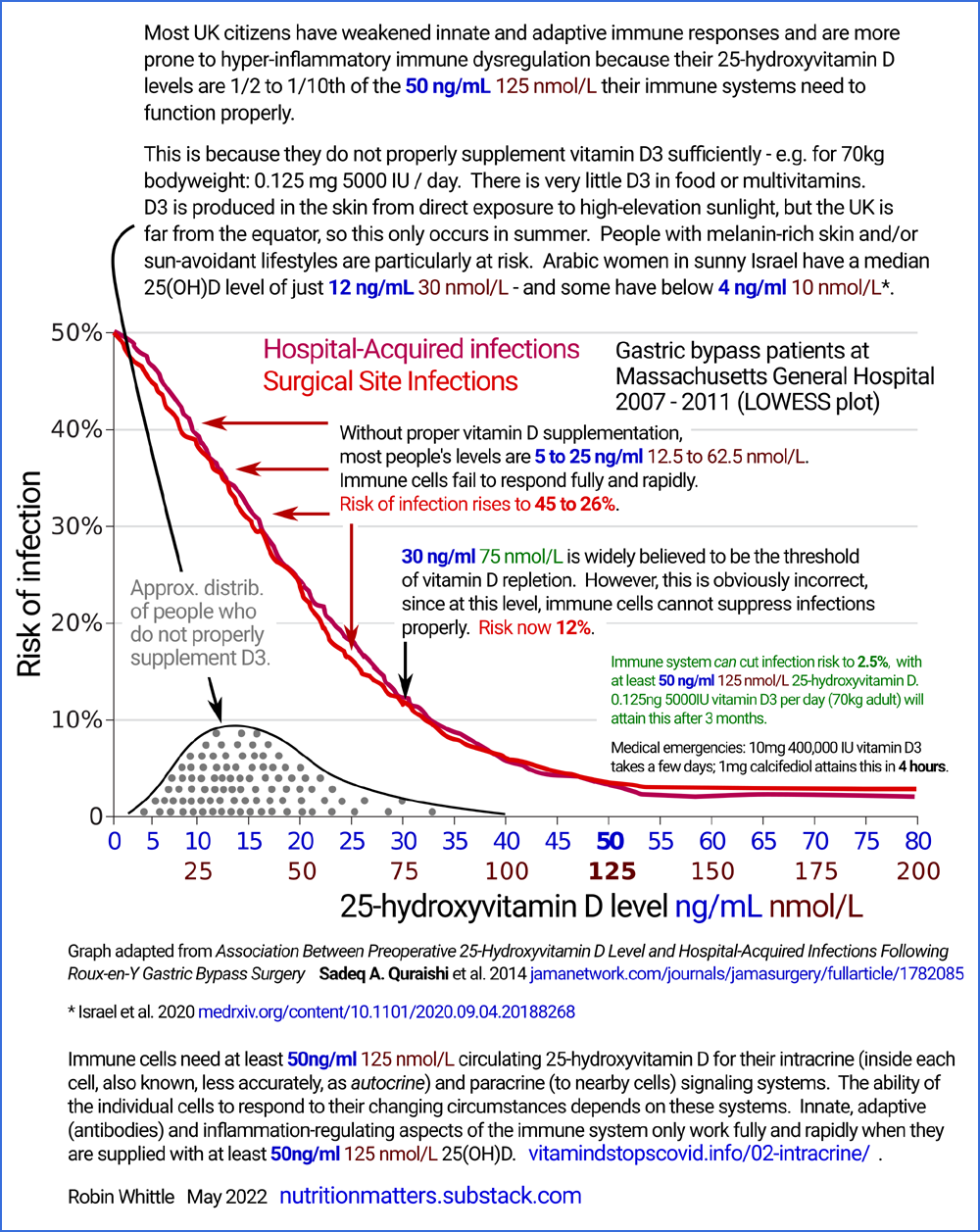
#04-quraishi
Quraishi et al. 2014: 25(OH)D requirements for immune cell
intracrine / paracrine signaling as indicated by hospital infection
rates
following surgery
Here is another way of understanding the need for proper 25(OH_D levels around or above
50 ng/mL (125 nmol/L).
The following graph comes from research into the risk of infections in
people (all morbidly obese) who had just been operated on for Roux-en-Y gastric
bypass [
WP]. weight loss surgery.
This is a weight-loss surgery
with numerous problems due to malabsorption of fats, iron, and other
nutrients including vitamin D3 and due to overly rapid, uncontrolled,
absorption of carbohydrates. It is a highly regarded operation in
the USA - I have not heard of anyone in Australia performing it.
However, less drastic operations such as gastric banding are gaining
favour over Roux-en-Y. All those who underwent this operation and
who were subjects in the Quraishi et al. retrospective analysis had
this operation to treat morbid obesity. This
seems crazy to me when they should
first try to reduce the imbalances which drive their obesity: robust
supplements for all micronutrients including especially vitamin D3, no
fructose, no caffeine and so less need for alcohol, nicotine and
anti-depressants / anxiolytics. However, morbid obesity is a
deadly medical problem and is very difficult to tackle - hence the
attraction of these drastic surgical interventions. Lack of
helminths is surely a significant factor in the metabolic and
inflammatory changes which contribute to obesity. Google Scholar: helminths obesity .
This PNG is from my Inkscape
version combining two similar graphs, made from the vectors in the
PDF. So the red and purple lines, and the scales, are direct from
the article's graphs, not a result of me trying to copy them by some
approximate method.
Please think of these graphs whenever you read of individual and
average 25(OH)D levels in people who are not adequately supplementing D3
and who do not get very substantial UVB skin exposure (which damages
DNA and increases the risk of skin cancer). Their levels are typically
between
5 and
20 or
25 ng/mL.
The graphs depict how the risk of infections in hospital - either
directly resulting from the surgery or due to other reasons - vary with
vitamin D 25(OH)D levels, for 770 patients.
Low rates of infections occur when the immune system's innate [
WP] and adaptive ([
WP]
antibodies etc. ) responses
are functioning properly. The high rate of infections at the left of the graph are due to weak immune
responses which directly combat the (primarily bacterial) pathogens
which cause these infections, as in the first intracrine
signaling example below. (The second Chauss et al. example
below concerns innate immune system regulatory lymphocytes which, when
their intracrine signaling fails due to lack of 25(OH)D, cause trouble by
producing pro-inflammatory cytokines for longer periods than they
should. This failure causes other immune cells to destroy healthy
cells, especially in the blood vessels of the lungs. This causes
or at least strongly drives severe COVID-19, but is not likely to be
important in the infections in hospital which are the subject of
Quraishi et al.'s research.)
With one potential exception,
wherever
the graphs rise above about 2.5%, this is due to vitamin D based intracrine / paracrine signaling
not working properly in some - probably many - types of immune cell. A potential exception is that that the higher D3 levels which give rise to the
higher 25(OH)D levels are also directly useful (without involving
intracrine / paracrine signaling) in the protection of endothelial cells [
Gibson et al. 2015].
I have not been able to quantify how important this is, and I suspect
the main cause of these infections is the failure of the innate immune
system to rapidly defeat bacteria.
The raised risks of infection indicate
dysfunction of intracrine / paracrine signaling due to inadequate
25(OH)D. Eyeballing this we see that the
40 ng/mL
minimum recommendations mentioned above don't go quite far
enough. The evidence of this substantial research (770 subjects,
in one hospital, with all the researchers being from Harvard Medical
School) indicates that we should be aiming for at least
55 ng/mL,
at least in these obese adults. There is some scatter in the
measurement of 25(OH)D levels. For this reason, and to simplify
things a little, I write of this research as if it suggests that at
last
50 ng/mL
is required for proper immune system function.
This research does not tell us directly what 25(OH)D levels are required
for Th1 regulatory lymphocytes to avoid the pattern of being stuck in
their pro-inflammatory startup program indefinitely, as described in Chauss et al. above
#chauss-1 above and
#chauss-2
below. There are surely other regulatory immune
cells which behave in a similar fashion - weakening direct innate and
adaptive responses or pathologically
over-strengthening inflammatory responses - if they don't have enough
25(OH)D. However, it is reasonable to guess that these Th1 cells
would generally work reasonably well only when 25(OH)D was also at or
above
50 ng/mL.
The exact 25(OH)D levels required to suppress hyper-inflammatory
immune responses surely vary considerably from one person the next,
according to individual genetic variation, in a context in which many
people have a problem with these responses, by way of auto-immune
inflammatory conditions, due to no longer having helminths.
Please see
https://vitamindstopscovid.info/06-adv/
for more information on this. I expect that if we all had
helminth infections, we would generally not need such high 25(OH)D levels
to suppress these autoimmune problems. This page links to mid-2021 research from Ethiopia which indicates that
active helminth infections reduce the risk of severe COVID-19 by 77%.
Helminths are known to suppress inflammatory responses, which are
primarily directed at helminths and other multicellular
parasites. They are not known to substantially suppress the
innate and adaptive responses to viruses, fungi or bacteria - and it is
primarily these anti-bacterial innate and adaptive responses which are
directly weakened by the lower than 50 ng/mL 25(OH)D levels which are
indirectly measured in Quraishi et al. by way of their weakening
increasing the risk of infection.
#05-intra
Description of vitamin D based intracrine signaling
The following description is mainly
of intracrine signaling, using vitamin D: 25(OH)D being converted to 1,25(OH)2D. Paracrine signaling is easy to understand
as an extension of this.
Step 1 - producing vitamin D receptor and 1-hydroxylase enzyme molecules
The diagrams below are adapted from a diagram in this 2011 article
which has a good description of vitamin D intracrine signaling in a particular type of immune cell, although
the term intracrine is not used:
Here is a description of intracrine signaling which assumes an interest
in cell biology, but little prior knowledge.
In this
example from Prof. Martin Hewison, we learn how toll-like receptors [
WP] on the
cell membrane of some types of monocytes [
WP]
- in this case a macrophage [
WP]
- respond to bacterial infections. The same principles of vitamin
D intracrine signaling apply to other types of cell, including the Th1
regulatory lymphocytes discussed below (Chauss et al. 2021), although
the
stimulus for activating intracrine signaling is totally different to
the bacterial fragments in the current example, and the response of the
lymphocyte is also entirely different.
In this cell type, fragments of pathogens activate toll-like receptors which are embedded [
WP]
in the cell membrane, with their detector section facing outwards and
other parts of the molecule facing inwards, immersed in the cytosol [
WP]. When the receptor is activated, it changes its shape so the part of the
molecule inside the cell causes some other signaling
molecules to migrate to the nucleus and upregulate the
transcription [
WP] of
two genes: for the 1-hydroxylase enzyme and for the vitamin D receptor (VDR) protein.
Those signaling molecules are cell-type specific, and somehow cause transcription enzymes to make mRNA (messenger RNA [
WP])
copies of the information in those genes. These multiple
mRNAs migrate out of the nucleus, to the cytosol, where they are found
by ribosomes [
WP]
which work along each mRNA molecule, following its instructions of which amino
acids to assemble into the protein chain. This is called
translation.
When each ribosome reaches the other end of the mRNA molecule, it
has
produced
one chain, which folds of its own accord to become a complete single
molecule of protein. This creates some number (I guess hundreds
or thousands)
of complete, operational, 1-hydroxylase enzyme molecules and
likewise vitamin D receptor molecules. (There can be other
details, such as editing the mRNA and modifications to the proteins.)
Step 2 - converting 25(OH)D to 1,25(OH)2D molecules and these binding to vitamin D receptor molecules
25(OH)D is carried in the blood plasma primarily bound to vitamin D binding protein molecules [
WP], with a lower proportion bound less strongly to albumin [
WP] proteins. (
#vdbp) A small proportion of these 25(OH)D molecules (
red
discs) are free to diffuse from the plasma, into the interstitial fluid
[
WP] between cells (in the case of cells which are not in the bloodstream or
in the walls of blood vessels) and then they diffuse
across the cells' lipid bilayer [
WP] plasma membrane into the cytosol of the cell.
Vitamin D3 cholecalciferol has only one hydrophilic hydroxyl group, in the 3 position with all its other
sides made up of hydrophobic hydrogen atoms. So it is soluble in oils but not
much in water. 25(OH)D has two hydroxyl groups and so is more
soluble in water.
Assuming there is an adequate concentration of 25(OH)D molecules (
red discs) - which there will be if blood levels are
50 ng/mL
or more, then it doesn't take long for one of these 25(OH)D molecules to
find its way to the active site of the newly created 1-hydroxylase enzyme molecules, be
hydroxylated at the 1 position, and be ejected back into the cytosol as 1,25(OH)
2D molecules, (
green discs).
By now there will be some number of vitamin D receptor VDR molecules and
the freshly made 1,25(OH)
2D molecules find their way (as described above
#thermal,
with random thermal motions and rotations) into the active site of one of these receptors, where
the two are strongly attracted and stick together as an activated
receptor complex.
This newly produced 1,1,25(OH)
2D is functioning as an intracrine agent which
binds to these vitamin D receptor molecules, inside the cell in which
the 1,25(OH)
2D was produced.
This step is identical for the vitamin D based intracrine signaling systems of all cell types.
Step 3 - Activated receptor complexes diffuse or migrate to the
nucleus where they alter gene transcription and so protein translation
When a 1,25(OH)
2D molecule binds to the
receptor molecule, this changes the shape of the receptor molecule and
causes some of them to migrate into the nucleus. (I guess only a
subset of them migrate to the nucleus, so perhaps is is diffusion,
rather than them all marching off in the direction of the
nucleus.) Articles mention them "translocating" to the nucleus,
but this just means "move " and I know of no active transport or
guidance system for them doing this, so for now I assume that the
activated 1,25(OH)
2D-VDR complexes simply move around at random, due to
thermal motion, and that some subset of them diffuse into the nucleus.
There the activated receptor complexes find their way
(by diffusion, I guess) to another molecule (retinoid X receptor [
WP], which is related to vitamin A and is not shown in these diagrams) with binds to them as well
and the entire heterodimer [
WP] complex then finds its way to particular
patterns of DNA which are exposed (according to how the DNA of the
various chromosomes are wrapped around and otherwise organised by
histones [
WP]) and ready to accept them. These are the VDRE (Vitamin D Response Elements [
WP])
and they are upstream of a particular gene which this process is
intended to increase or decrease the copying of. (The whole human genome
has thousands of such genes, with one or more VDREs upstream. By various
means, each cell type exposes only these to being bound by the
heterodimer complex - the particular genes which this cell needs to be
copied in order to respond to its circumstances properly.)
Once the VDRE section of DNA has the heterodimer attached, in some
circumstances this
signals DNA copying enzymes to start work there, copying the data in
the downstream gene into messenger RNA molecules. In others, it
reduces the amount of copying of this gene. (I have found some
explanations of these mechanisms but they are exceedingly complex and
require a strong understanding of DNA, histones and the
like. I think these low level details don't need to be
understood in order to have a good general understanding of vitamin D
based intracrine and paracrine signaling.)
In principle, if this process of activated receptor complexes finding
their way to these transcription regulator molecules was highly guided,
then there would only need to be a handful of 25(OH)D molecules converted
to 1,25(OH)
2D, perhaps by a single or a few enzyme molecules, and likewise
there would only need to be a handful of vitamin D receptor molecules.
However, since (as far as I know) the processes are unguided
(diffusion) or at least not
very efficient, and since the activated complexes and probably the 1,25(OH)
2D molecules would have relatively short half-lives (of their own
accord, or by 24-hydroxylase enzymes changing them into molecules which are broken down, to get rid of them once the
conditions which activated intracrine signaling no longer occurred) then
there needs to be quite a quantity of both 1,25(OH)
2D and vitamin D
receptor
molecules ready to bind together in order to make a substantial and
sustained change in gene transcription, which is required for the
behaviour of the cell to change significantly. This requires
continual
conversion of 25(OH)D to 1,25(OH)
2D, since the 1,25(OH)
2D molecules have
relatively short half-lives. As noted above, to fully alter the
gene translation process to change the cell's behavior, it seems there
needs to be around
1 ng/mL (1 part per billion by mass) 1,25(OH)
2D in the cytosol of the cell.
Considering that:
- There an unknown number of cell types using vitamin D
for intracrine signaling.
- There are likely countless billions
of cells of each such cell type.
- The intracrine signaling systems of each cell of these types is activated
at least some of the time .
- Only a subset of the 25(OH)D is
actually used by intracrine / paracrine signaling, one might expect the total vitamin D3
requirements per year to supply this 25(OH)D to be substantial. But
0.045 grams per year is all it takes for 70 kg adults to maintain, on average, about 50 ng/mL
25(OH)D - and only about 1/2 or 1/4 of ingested or UV-B produced D3
molecules wind up as circulating 25(OH)D molecules after hydroxylation in
the liver.
With upregulated gene copying, the newly copied mRNA molecules
leave the nucleus and go into the
cytosol, where ribosomes run along them, making the proteins they
contain the instructions for. (For downregulation, fewer of these
mRNA molecules are produced than previously.) These proteins are
the ones which
make the cell respond to its changed circumstances. In some
cells, these may be exported to kill pathogens, or to kill infected
cells. In others, the proteins may cause the release of
pro-inflammatory or anti-inflammatory cytokines [
WP] - signaling molecules which control activities of other types of immune cell which are nearby.
The alterations to gene
transcription alter the mix of mRNAs in the
cytosol and so (
translation) the quantities of proteins produced by the
ribosomes which run along them. (mRNAs have quite short lives, so
for continual protein production a continual supply of them via
transcription is required.)
This altered set of protein products is what drives the cell to alter
its behaviour. In this example, the altered behaviour sets the
cell up for engulfing and digesting bacteria.
In the Chauss et al. 2021 example below
#chauss-2, when the intracrine signaling
systems of a Th1 lymphocyte is activated and works properly, the
lymphocyte switches from its pro-inflammatory startup program to its shutdown program in which it produces less
of a pro-inflammatory cytokine and more of an anti-inflammatory
cytokine.
Paracrine signaling
One part of paracrine signaling is
depicted at the bottom left of the above diagrams: some of the newly
produced 1,25(OH)
2D diffuses out of the cell and reaches nearby
cells.
As noted above
#02-nothorm, the concentration of this 1,25(OH)
2D inside the cell in which it is produced is probably around
1 ng/mL
(1 part per billion by mass) when the intracrine signaling system is is fully
activated and there is sufficient 25(OH)D to feed the hydroxylation process. This
1 ng/mL is much higher than the very low levels of 1,25(OH)
2D present in the bloodstream
as a hormone to regulate calcium-phosphate bone metabolism: around
0.045 ng/mL (0.045 parts per billion).
The newly produced 1,25(OH)
2D is functioning as a paracrine agent when it
diffuses out of the cell, and makes its way to other nearby cells where
- by one means or another - this increased local level of 1,25(OH)
2D is
detected in a way which alters the behaviour of those nearby
cells. As far as I know, VDR is only found in the cytosol and
nucleus of the cell - it is not located in the cell membrane, ready to
detect 1,25(OH)
2D outside the cell. So, as far as I know, paracrine
signaling works by extracellular 1,25(OH)
2D, diffused from where it was
produced in nearby cells, diffusing into the cytosol and binding to VDR
molecules there. Then, some of the bound complexes diffuse into
the nucleus and alter cell behavior as just described for intracrine
signaling.
I have not read a detailed account of vitamin D based paracrine
signaling. I guess the distances might be 1 mm to 10 mm or so, but
if there was a particular part of the body, such as a 5 cm sphere, in
which a population of some immune cell of type A generally has all its
cells in a state of activated and successful paracrine signaling (or at
least producing 1,25(OH)
2D purely for paracrine purposes) than these could
collectively flood the area with 1,25(OH)
2D as a paracrine agent and so
alter the behaviour of a population of cells of some other type B in
that area. So while a lone cell releasing 1,25(OH)
2D as a paracrine
agent into the bloodstream or fluid between cells in an organ wouldn't
be able to raise the concentration very much except over a very short
distance from itself of (tens of microns??) with a large number of such
cells, their collective output could use paracrine signaling over a
greater distance.
#chauss-2
The Chauss et al. article on intracrine signaling failing in Th1 lymphocytes due to lack of 25(OH)D
If most or all of the above makes sense
to you, then you are in a good position to either read the entire
Chauss et al. 2021 article, or at least my summary and discussion of
its preprint, at:
The basic summary is below. The above page has a more extensive summary and discussion.
Then, you will have a real, cellular and molecular level understanding
of some of the most important reasons why the world is going to hell in
a handbasket at present (first written in November 2020, and still
en-route in May 2022, though Omicron has been a blessing due to its
significantly reduced virulence compared to Delta and previous
variants). Some people are still getting sick from
COVID-19, and I assume that 10 million people a year, at least, are
still being killed by sepsis
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)32989-7.
It would be much easier if everyone took vitamin D supplements to raise
their 25(OH)D levels to the
50 ng/mL or more levels which enable our vitamin D
intracrine and paracrine signaling systems to work properly.
Autocrine vitamin D signaling switches off pro-inflammatory programs of Th1 cells
Daniel Chauss, Tilo
Freiwald, Reuben McGregor, Bingyu Yan, Luopin Wang, Estefania
Nova-Lamperti, Dhaneshwar Kumar, Zonghao Zhang, Heather Teague, Erin E.
West, Kevin M. Vannella1, Marcos J. Ramos-Benitez, Jack Bibby, Audrey
Kelly1, Amna Malik1, Alexandra F. Freeman, Daniella M. Schwartz, Didier
Portilla1, Daniel S. Chertow, Susan John, Paul Lavender, Claudia
Kemper, Giovanna Lombardi, Nehal N. Mehta, Nichola Cooper1, Michail S.
Lionakis, Arian Laurence, Majid Kazemian and Behdad Afzali
Nature Immunology 2021-11-11
https://www.nature.com/articles/s41590-021-01080-3
|
I regard this article as
the most important article in the entire COVID-19 literature.
This is my best attempt to describe some complex processes I have no expertise in.
Th1 lymphocytes isolated from the lungs of patients with
severe COVID-19 symptoms have an intracrine signaling pathway
(in this article, referred to as "autocrine") which should be
activated by high levels of complement (WP), to turn these cells off their initial hyper-inflammatory
startup program which produces primarily pro-inflammatory IFNγ (interferon_gamma WP
which has antiviral and anti-bacterial activity as well as stimulating
inflammation: cell destruction such as by natural killer cells WP) and instead cause them to produce primarily the anti-inflammatory cytokine IL-10.
(The cells always produce both these cytokines, but this transition to
a shutdown, anti-inflammatory program, involves less IFNγ and a lot
more IL-10.)
However,
this anti-inflammatory pathway is not working in the Th1 cells from patients with severe COVID-19, due largely or solely to insufficient 25,hydroxyvitamin D3 == 25(OH)D == calcifediol for each cell's intracrine signaling system to function.
This is a molecular and cellular explanation for why people with
low vitamin D have wildly dysregulated, overly-inflammatory (cell
killing), self-destructive immune responses. Such responses drive
sepsis, severe influenza, Kawasaki disease (KD WP), Multisystem Inflammatory
Syndrome (MIS discussion) and of course severe COVID-19. (See Paul Marik's explanation https://www.evms.edu/covid-19/covid_care_for_clinicians/ of how it is the immune response, not the virus, which causes the escalation to severe symptoms and death. See https://aminotheory.com/cv19/#2015-Stagi for research which shows KD children have very low 25OHD vitamin D levels.)
In severe COVID-19, severe inflammation in the lungs damages
endothelial cells (the inner lining of blood vessels and capillaries WP)
leading to hypercoagulative blood, causing microembolisms and larger
clots all over the body, which cause most of hypoxia, lasting harm and
death.
It is not known whether the cause of all
the hyper-inflammatory immune system dysregulation - which causes some
COVID-19 sufferers people to develop
severe symptoms - is primarily the failure of these Th1 lymphocytes to
switch from being pro-inflammatory to anti-inflammatory,
or whether this endothelial cell destruction etc. is also driven to a
significant degree by similar failures in the intracrine signaling
systems of many other
types of regulatory and/or directly anti-pathogen immune cell.
However, the determination of
the exact mechanism of failure in Th1 cells, in the context of such failures likely
also occurring in other types of immune cell, is an extraordinarily valuable
contribution which needs to be very widely understood.
No-doubt
the virus on its own is causing destruction in the lungs and elsewhere,
so I don't want to portray all harmful outcomes of COVID-19 as
resulting from the immune system's overly inflammatory response.
For instance,
Wenzel et al. 2021
describes a viral enzyme cleaving a human protein NEMO which is
essential to brain endothelial cells, resulting in blood brain barrier
disruption and the death of capillaries, to become "string
vessels". This seems not to be related to inflammatory responses,
but is a good argument for proper 25(OH)D levels and early treatments to
enable the body to tackle the virus ASAP, hopefully before such
terrible things happen to our brains.
Low
vitamin D levels (low circulating 25(OH)D, produced in the liver
from UV-B-generated and/or ingested vitamin D3 cholecalciferol) are
well known to reduce the effectiveness of numerous direct,
anti-pathogen, responses by the innate immune system cells and to
hinder the creation of antibodies for adaptive immune responses. These
immune functions of vitamin D 25(OH)D are due to it being needed, in the
circulation, at higher levels than are sufficient for bone health
(sufficient for the kidneys to produce their much lower concentration
of circulating - and so hormonal - 1,25(OH)2D),
to
supply the intracrine / paracrine (inside the cell / to nearby cells)
signaling systems of all types of immune cells. Most or all types
of immune
cell can express the vitamin D receptor - and this is for intracrine /
paracrine signaling - not for responding to the much lower
levels of circulating1,25(OH) 2D which regulates
calcium-bone metabolism. #02-nothorm .
This McGregor et al. article is a beauty. It gets
down to brass tacks with the molecular processes of one aspect of the
cytokine storm of immune dysregulation which is crucial to the
development of severe COVID-19.
This failure may be the the
biggest single pathological process which causes some people to develop
severe COVID-19 symptoms. If not, then the similar failures of intracrine signaling systems in all other immune cells would be the
primary explanation. Surely the same dysfunction drives sepsis and many other conditions involving excessive inflammation.
This is one example of a specific vitamin D intracrine signaling system
in one particular type of immune cell. The following article
discusses the evolutionary basis and other details of
189 human genes know to be regulated in an intracrine / paracrine manner by vitamin D in monocytes [
WP], which are a subset of leukocytes [
WP],
which are a subset of immune cells which are a subset of the cell types
in the body which use their own particular version of vitamin D intracrine / paracrine signaling.
I don't want to imply that I have read
this, or that I would be able to understand it without weeks of
work. I cite it as an easy way of expanding upon this one
concrete example which surely plays a crucial role in severe COVID-19
(and see
https://aminotheory.com/cv19/#2020-Castillo for how oral
25(OH)D == calcifediol for hospitalised patients causes most of them to get much better,
very quickly) to indicate how important this general principle of
vitamin D intracrine signaling is.
Why so complex?
Intracrine signaling is quite
complex. However, there are plenty of other biochemical processes
which are more complex - for instance the citric acid cycle [
WP]
Inquiring minds want to know why
this evolved and is used for so many cell types. This is a Mouse Trap (
video) approach to biology - Rube Goldberg [
WP]
engineering when we can imagine something simpler would do
the job. This wacky complexity is a feature of biology - and some
or many of the idiosyncratic features of the evolved systems give rise
to valuable mechanisms.
Why, for instance,
doesn't the sensing of the changed condition lead to direct
upregulation and downregulation of whatever genes the cell needs to
produce (or no longer produce) the proteins which will make respond as
it is meant to?
Sidebar for the really curious:
I guess
the answer might be that the evolved capacity of the activated vitamin
D receptor (a single VDR molecule bound to a 1,25(OH)2D molecule) to
upregulate and downregulate multiple genes turned out be flexible and
useful. The activated receptor complex binds to particular gene
transcription promoter patterns (VDREs) in the DNA which are upstream
of the genes whose rate of copying to messenger RNA is to be up- or
down-regulated.
This flexibility and ability to alter multiple genes at once, and to
make these genes different from one cell type to the next - such as by
having different histone arrangements to mechanically expose different
parts of chromosomes and so genes - may have
some advantages over the simpler arrangement of, for instance, whatever
signaling system enables an activated toll-like receptor [WP]
to alter gene transcription (a first step in intracrine signaling)
somehow evolving flexibility over multiple types of cell to alter as
many genes in any one cell type, and in so many cell types, as are
altered by the activated vitamin D receptor.
Just to keep us on our toes, in his article, Martin Hewison describes a
separate set of processes operating in parallel to this intracrine
signaling system, also driven by the activation of the toll-like
receptors, which turns on some other aspects of the cell's response.
See also Martin Hewison's article PMC2854233
regarding how the vitamin D intracrine signaling we humans have is
specific to primates, and not found in rodents and other families of
mammals. He estimates this approach to intracrine signaling is
about 40 million years old.
Please also see this article suggesting some hypotheses about the
long-term evolutionary history of the vitamin D compounds and their
receptors, enzymes and binding proteins.
I roughly understood it up to about page 6.
© 2020 to 2022 Robin Whittle Daylesford, Victoria, Australia